Thermodynamics is the branch of physics that deals with the relationships between heat, work, temperature, energy, and the properties of substances. The fundamental laws of thermodynamics are:
The Zeroth Law of Thermodynamics:
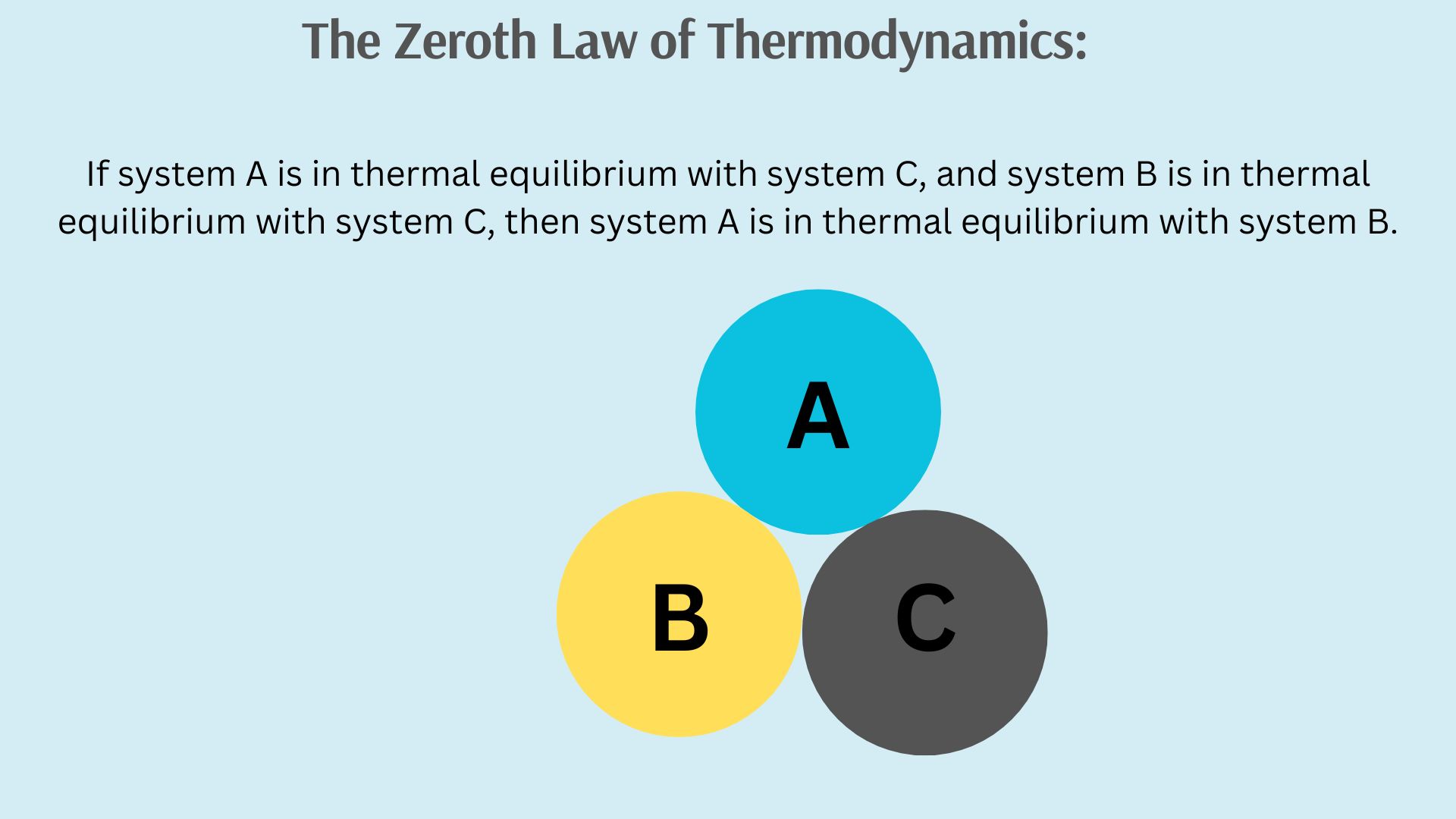
The Zeroth Law of Thermodynamics is a fundamental principle that establishes the concept of temperature and thermal equilibrium. It states that if two systems are each in thermal equilibrium with a third system, then they are also in thermal equilibrium with each other. In simpler terms, if two objects are at the same temperature as a third object, they are also at the same temperature as each other.
This law is called the “Zeroth Law” because it was formulated after the First and Second Laws of Thermodynamics were already established, hence the numbering as “zeroth.” The significance of the Zeroth Law lies in its role in defining temperature and providing a basis for the measurement of temperature. It allows us to compare the thermal states of different systems and understand how heat flows between them. For example, it’s the reason why a thermometer works—it equilibrates with the object being measured, indicating their thermal equilibrium.
This law is foundational in understanding the behavior of thermodynamic systems and plays a key role in the development and application of thermodynamics principles in various scientific and engineering fields.
The First Law of Thermodynamics OR Law of Energy Conservation:
The First Law of Thermodynamics, also known as the Law of Energy Conservation, is a fundamental principle in thermodynamics that deals with the conservation of energy within a system. It states that energy cannot be created or destroyed; it can only change forms or be transferred from one form to another.
Mathematically, the First Law of Thermodynamics is expressed as:
ΔU=Q−W
Where:
- ΔU is the change in internal energy of the system,
- Q is the heat added to the system,
- W is the work done by the system.
This equation signifies that any change in the internal energy of a system is equal to the heat added to the system minus the work done by the system. In other words, the internal energy of a system can increases if heat is added to it or work is done on it, and it can decrease if the system releases heat or does work.
The First Law has several implications and applications across various fields:
- Conservation of Energy: It confirms the principle that energy is conserved within a closed system, leading to the understanding that energy transformations are fundamental but always balanced.
- Heat and Work Interaction: It defines how heat and work interactions affect the internal energy of a system, leading to the development of concepts like heat engines, refrigerators, and heat pumps.
- Thermodynamic Processes: It helps analyze and understand different thermodynamic processes, such as isothermal, adiabatic, and isobaric processes, by quantifying the energy changes involved.
- Energy Transfer: It explains how energy is transferred between systems and how energy conversion processes occur in various devices and systems.
The Second Law of Thermodynamics:
It can be stated in several different ways based upon various observations. Two most common statements are given below:
Clausius Statement: This statement focuses on the heat transfer aspect of the Second Law. It states that heat cannot spontaneously flow from a colder body to a hotter body without external work being done on the system. In other words, heat naturally flows from hot to cold, and to reverse this flow, work must be applied.
Kelvin-Planck Statement: It is impossible to construct an Engine working on cyclic process whose sole purpose is to convert all the heat energy supplied to it to an equivalent amount of mechanical work. In other words, no engine in cyclic process can convert all the heat energy supplied to it into mechanical work. It means that some of the heat energy must be lost to the surroundings.
The Second Law of Thermodynamics has several important implications:
- It explains why certain processes are irreversible, such as heat flow from hot to cold, and why perpetual motion machines (machines that operate without external energy input) are impossible.
- It guides the design and operation of heat engines, refrigerators, and other thermodynamic systems by setting limits on their efficiency.
- It provides a basis for understanding the arrow of time and the directionality of natural processes.
Overall, the Second Law is a cornerstone of thermodynamics and plays a crucial role in shaping our understanding of energy transfer, efficiency, and the behavior of physical systems.
“MechView: Unveiling Mechanical Engineering, Simply Explained!”