Engineering materials:
Engineering materials refer to substances or compounds that are specifically designed and used for various engineering applications. These materials are selected based on their properties, such as strength, durability, conductivity, and thermal resistance, to meet the requirements of a particular engineering project. Engineering materials can be broadly categorized into metals, non-metals, polymers, ceramics, and composites. Each type of material has its unique characteristics and advantages, making it suitable for specific engineering purposes.
Classifications of engineering materials:
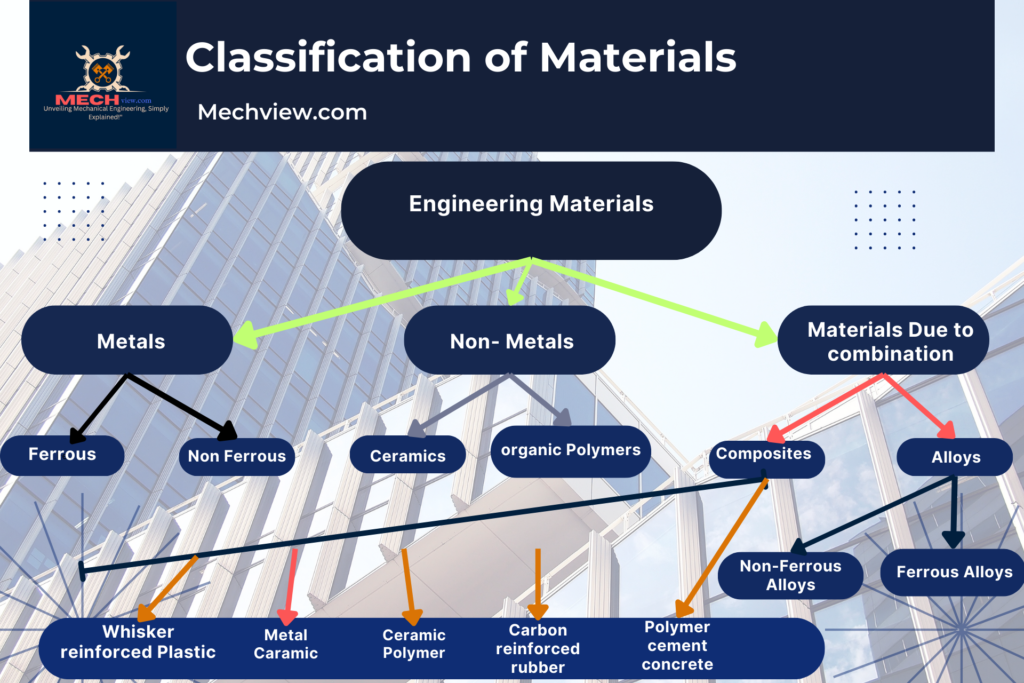
- Metals: Metals are defined as the substances which can easily give up or loose electrons to form metallic bonds with other substances. Medals have clouds of free electrons.
Metals are a group of materials characterized by their properties such as high electrical and thermal conductivity, malleability, ductility, and opacity to light. These materials are typically solid at room temperature (except for mercury) and have metallic luster. Metals have been essential to human civilization for millennia, playing a crucial role in technology, construction, manufacturing, and various other industries.
Characteristics of Metals:
- High electrical conductivity.
- High thermal conductivity.
- Malleability (ability to be hammered or pressed into shape without breaking).
- Ductility (ability to be stretched or drawn into thin wires).
- Luster or shine on the surface.
- High melting and boiling points.
- Good strength-to-weight ratio.
- Corrosion resistance in certain metals.
- Versatility in various applications, including construction, manufacturing, and electrical industries.
Application of Metals:
- Construction: Used in building structures, roofing, cladding, and piping.
- Manufacturing: Utilized in machinery, tools, equipment, and automotive parts.
- Electrical and Electronics: Employed in wiring, cables, electronic components, and circuitry.
- Transportation: Essential for vehicles, airplanes, ships, and railway tracks.
- Packaging: Used in cans, foils, and packaging materials.
- Medical: Used in medical devices, implants, and instruments.
- Energy: Used in energy generation, batteries, wind turbines, and solar panels.
- Jewelry and Ornaments: Utilized in jewelry and decorative ornaments.
The metals may be of ferrous non- ferrous type:
a). Ferrous metals: these metals contain iron as the main constituent with a varying proportion of carbon and other materials like sulphur, manganese etc. to enhance the various properties desired.
Some examples of ferrous metal are as the following: mild steel, medium carbon steel, high carbon steel, cast iron.
b). Non-Ferrous metals: These metals do not contain iron and carbon as their main constituents. A small proportion of iron and carbon may be present.
Aluminium, zinc, copper, silver, nickel, tin, chromium etc. are some main examples of known ferrous metal.
2. Non-Metals: Non-metals are defined as the substances which have the tendency to gain two or more electrons. They are also good thermal and electrical insulators. These materials are generally brittle.
Non-metals are elements that don’t have the typical characteristics of metals, like being shiny or good conductors of heat and electricity. They can be gases, liquids, or solids, and they’re used in various ways in industries and everyday life.
Characteristics of non-metals:
- These are bad conductor of heat and electricity.
- They have no luster.
- These are non-ductile and non-malleable.
- These have a tendency to gain one or more electrons.
- These are brittle solids at room temperature.
- These have excellent resistance to corrosion and oxidation.
Depending upon their behavior and application the non-metals may be following types:
1. Ceramics: Ceramics are the materials made by the mixture of metal and non-metal these are generally the oxides, carbides, nitrites, borides, silicates of metal and non-metals. These have high hardness inertness and abrasion resistance.
Rocks, glasses, fireclay, and firebricks, cement and lime are ceramics.
2. Composites: These materials are manufactured by combining more than one different material. These are specially designed to have best property of two materials. Cemented carbide, fibre in fibre glass is an example of composite materials.
These are used in refrigerating unit’s aircrafts structures electric transmission towers etc.
3. Organic polymers: Organic polymers are relatively inert and light and generally have a high degree of plasticity. These are derived mainly from the hydrocarbons. These consist of covalent bond formed by carbon, chemically combined with oxygen and hydrogen. Thus, polymers are obtained from monomers bonded by chemical reaction possess called polymerization.
These materials can be further sub classified as thermosetting and thermoplastic material.
Difference between metal and non-metal:
| Characteristic | Metals | Non-Metals |
|---|---|---|
| Electron accepting/ losing tendency | Medals have tendency to lose electrons | Non- metals have tendency to gain electrons |
| Physical Properties | High melting points, high density, luster | Lower melting points, lower density, dull |
| Physical State | These are generally solid (Hg is exception) | These are solids liquid and gases |
| Appearance | Shiny or metallic luster | Dull or non-metallic appearance |
| Malleability/Ductility | Malleable and ductile | Brittle, lack malleability and ductility |
| Conductivity | Good conductors of heat and electricity | Poor conductors of heat and electricity |
| Reactivity | React with acids, undergo corrosion | React with metals, form acidic oxides |
| Melting point and boiling point | High | Low |
| Examples | Iron, copper, aluminum | Carbon, oxygen, nitrogen |
| Uses | Construction, electrical wiring | Electronics, construction materials, fuels |
“MechView: Unveiling Mechanical Engineering, Simply Explained!”